What is the carbonate paradox?

Calcium- and magnesium-containing carbonate deposits (limestones) are abundant on Earth, totaling approximately 40 million gigatons, and account for more than half of the chemical forms of carbon on the planet. Most of these limestones originate from biominerals produced by marine organisms. Biominerals serve various biological functions, such as structural support, protection, mineral storage, and gravity sensing. The process by which these biominerals are formed is called biomineralization. For example, the shells of mollusks, the skeletons of corals, the skeletons of sea urchins, and the hard tissue of foraminifera—planktonic or benthic unicellular eukaryotes—are biominerals composed of calcium carbonate (CaCO₃), a type of carbonate.

In recent years, the increase in atmospheric carbon dioxide (CO₂) concentration due to human activities has
triggered various environmental changes, including global warming and ocean acidification. In this context,
a variety of CO₂ sequestration strategies have been explored. Because many biominerals are carbonate
minerals, they would appear to be closely related to CO₂ fixation. However, the process of CO₂ fixation
through biomineralization has received little attention to date.
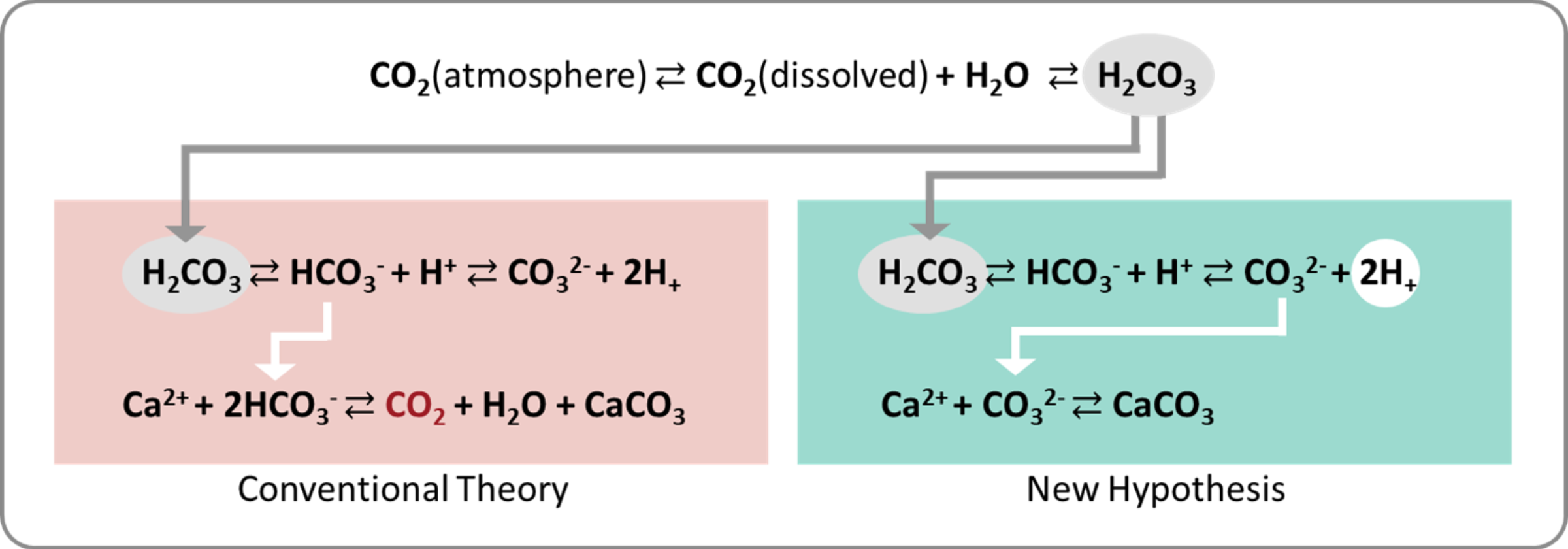
When CO₂ dissolves in seawater, it hydrates to form H₂CO₃, which then dissociates immediately into H⁺ and
HCO₃⁻. The presence of these protons (H⁺) is the reason why CO₂ dissolution lowers pH, causing so-called
ocean acidification. Some HCO₃⁻ further dissociates into H⁺ and CO₃²⁻, but because seawater has a pH of
around 8, most inorganic carbon remains in the form of HCO₃⁻.
In biological systems, Ca²⁺ ions in seawater react with HCO₃⁻ to form calcium carbonate (CaCO₃), water (H₂O),
and carbon dioxide (CO₂). This reaction also releases protons (H⁺), lowering pH further. The drop in pH
shifts the carbonate equilibrium toward the generation of CO₂, which is ultimately released back to the
atmosphere. For this reason, the formation of CaCO₃ through biomineralization has long been considered
unsuitable as a CO₂ sequestration strategy.
――However, is this really the case? If the biomineralization process were a reaction system that releases
CO₂, it would contradict the well-established fact that a vast amount of carbon at the Earth's surface is
deposited as biomineral-derived limestone. We refer to this contradiction as the “carbonate paradox.”
Recent studies have reported cases in which the pH within organisms actually increases during biomineral
formation. This behavior is contrary to the biological reaction systems where CO₂ release causes a pH decrease.
Furthermore, it has become clear that specialized biomineral proteins catalyze the reaction between Ca²⁺ and
CO₃²⁻, thereby promoting calcification within living organisms. These findings suggest the presence of
kinetic control mechanisms in vivo that cannot be explained solely by equilibrium chemistry of CO₂
dissociation.
By integrating knowledge from environmental science, geology, mineralogy, and biology, we aim to precisely
visualize the biomineralization process—specifically, how calcium and carbonate are transported, how calcium
carbonate is deposited through the action of organic matrices, and how protons are metabolized within living
organisms—and thereby address the resolution of the “carbonate paradox.”
